Why Use Silicon Bronze?
Bronze has been the material of choice for boat fittings for centuries. It is resistant to most common causes of corrosion and strength makes it the ideal alloy for cleats, fairleads, chocks, rowlocks and many other items commonly found on ships.
-
Silicon bronze is more resistant to water corrosion than brass, making it highly useful for marine applications.
-
It is resistant to freshwater, saltwater, fog/mist, alkalis, acids, organic chemicals, and is only susceptible to attack via sulfides, nitric acid, chromates, ferric chloride, and other oxidizing salts.
-
Silicon bronze is anti-biofouling.
-
Silicon bronzes stand above traditional alloys as an exceptionally resistant, yet handsome material for marine applications.
For Further Information - All About Silicon Bronze
Alternative Materials for Fasteners
There are numerous different materials that can be used for manufacturing fasteners. Commonly used examples and the potential issues of utilising them within the yacht are illustrated below.
Stainless Steel
Grade 304 and 316 stainless steels (A2 and A4 respectively) are known for the all-round corrosion resistance and is usually the go to material in the marine environment due to this common misconception. The traditional ferritic and austenitic stainless steels (including grades 304 and 316) are potentially unsuitable for use in seawater due to being prone to crevice corrosion and pitting attack, giving rise to a high probability of premature failure.
Stainless steels require constant exposure to oxygen to maintain a chromium oxide film on the surface. This protects the base material and makes it corrosion resistant. Inserting 316 stainless steel fasteners through a deck or other tight cavity that can prevent exposure to air, and water makes contact, the fasteners can be susceptible to crevice corrosion.
Why Can Stainless Steel Corrode?
To work at its best, stainless steel needs free access to oxygen. A crevice is a narrow gap between a piece of metal and another piece of metal or tightly adhering material like plastic or a film of bacterial growth. Crevices are wide enough to permit entry of moisture but narrow enough to prevent free circulation. A perfect example of this situation is recessed and plugged fasteners in hull planking.
The result is that the oxygen in the moisture is used up. In addition, if chlorides (i.e. those found in salt water) are present they can concentrate in the stagnant conditions and the moisture can become acidic. These are all conditions that can lead to the breakdown of the passive film on the stainless steel. Attack can then progress rapidly, and eventual failure of the fastener can occur.

Figure 1 - Crevice Corrosion of AISI 316L Tube Crevice Corrosion
Disadvantages of Stainless Steel on a Yacht
-
Due to grades 304 and 316 stainless steel being further apart on the galvanic series table than silicon bronze from both cast iron and lead (usual ballast material), the corrosion rate of the sacrificial anodes will be greatly increased, when used below the water line, potentially resulting in the anodes needing to be replaced more often.
-
Utilising stainless steel below the waterline will potentially lead to significant corrosion of all components containing stainless steel and will need to be replaced prematurely.
For Further Information - British Stainless Steel Association (bssa.org.uk)
Brass
Brass is an alloy of copper and zinc, in proportions which can be varied to achieve varying mechanical and electrical properties.
For anyone who has tried to remove old brass screws from a project, they will know the thought of hoping that the screws will not sheer off. Although brass will not rust or corrode in the same way as stainless steel, brass will undergo corrosion by different means. Dezincification is a process which selectively removes zinc from an alloy, leaving behind a porous, copper-rich structure that has little mechanical strength causing the sheering of the brass upon applying a torque to the fastener.
Dezincification can show itself in a variety of ways depending on the water composition and service conditions. It may present itself as dull red spots on the surface of brass. Extreme dezincification can cause actual breakage, with a dull coppery appearance to the fracture surface.

Figure 2 - Dezincification of Brass Screws to Leave Brittle Remnants
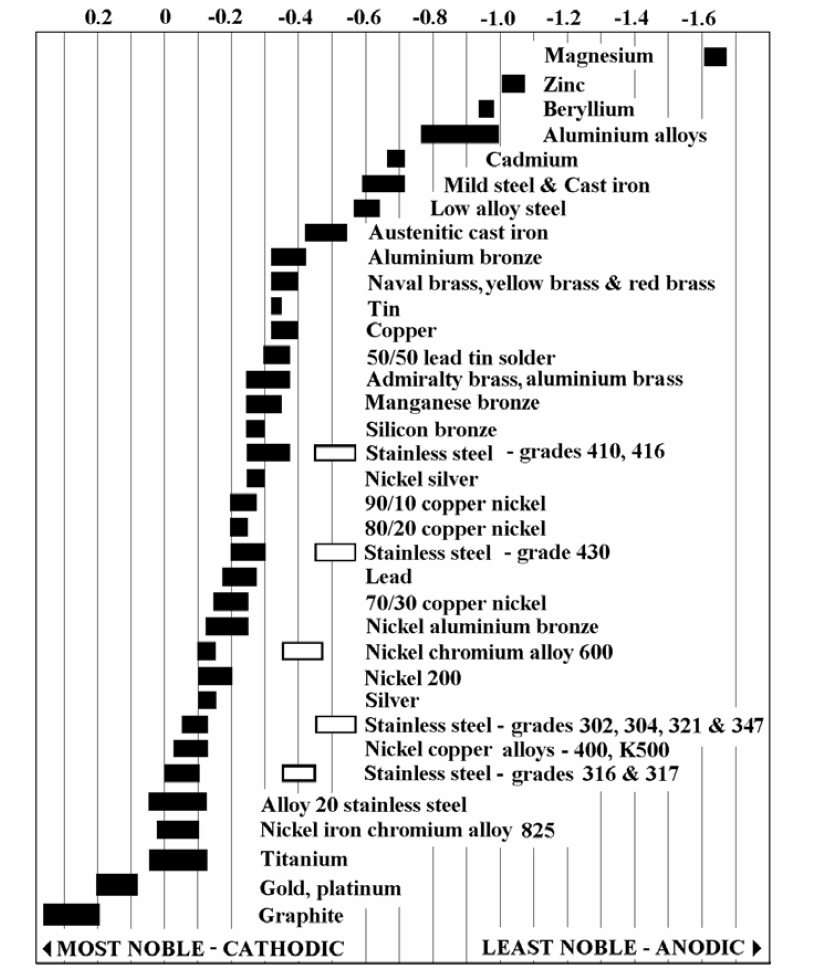
Galvanic Series
The galvanic series (or electropotential series) determines the nobility of metals and semi-metals. When two metals are submerged in an electrolyte (for example seawater), while also electrically connected by some external conductor, the less noble (base) will experience galvanic corrosion. The rate of corrosion is determined by the electrolyte, the difference in nobility, and the relative areas of the anode and cathode exposed to the electrolyte. The difference can be measured as a difference in voltage potential: the less noble metal is the one with a lower (that is, more negative) electrode potential than the nobler one, and will function as the anode (electron or anion attractor) within the electrolyte device functioning as described above (a galvanic cell). Galvanic reaction is the principle upon which batteries are based.
Figure 3 - Galvanic Series Chart